General Information/IS and Gene Expression: Difference between revisions
No edit summary |
No edit summary |
||
| Line 17: | Line 17: | ||
IS activity can affect efflux mechanisms resulting in increased resistance: [https://tncentral.ncc.unesp.br/ISfinder/scripts/ficheIS.php?name=IS1R IS''1''] or [https://tncentral.ncc.unesp.br/ISfinder/scripts/ficheIS.php?name=IS10R IS''10''] insertion can up-regulate the [[wikipedia:Efflux_(microbiology)|AcrAB-TolC]] pump in ''[[wikipedia:Salmonella_enterica|Salmonella enterica]]''<ref name=":6"><pubmed>15616308</pubmed> | IS activity can affect efflux mechanisms resulting in increased resistance: [https://tncentral.ncc.unesp.br/ISfinder/scripts/ficheIS.php?name=IS1R IS''1''] or [https://tncentral.ncc.unesp.br/ISfinder/scripts/ficheIS.php?name=IS10R IS''10''] insertion can up-regulate the [[wikipedia:Efflux_(microbiology)|AcrAB-TolC]] pump in ''[[wikipedia:Salmonella_enterica|Salmonella enterica]]''<ref name=":6"><pubmed>15616308</pubmed> | ||
</nowiki></ref>; [https://tncentral.ncc.unesp.br/ISfinder/scripts/ficheIS.php?name=IS1R IS''1''] or [https://tncentral.ncc.unesp.br/ISfinder/scripts/ficheIS.php?name=IS2 IS''2''] insertion upstream of AcrEF<ref name=":7"><pubmed>11302812</pubmed></nowiki></ref><ref><nowiki><pubmed>11274125</pubmed></nowiki></ref> and [https://tncentral.ncc.unesp.br/ISfinder/scripts/ficheIS.php?name=IS186 IS''186''] insertional inactivation of the AcrAB repressor, AcrR, in ''[[wikipedia:Escherichia_coli|Escherichia coli]] <ref name=":7" />'', all lead to increased resistance to [[wikipedia:Quinolone_antibiotic|fluoroquinolones]]. Insertional inactivation of specific [[wikipedia:Porin_(protein)|porins]] can also play a significant role<ref><nowiki><pubmed>15212803</pubmed></nowiki></ref>.<center>[[Image:1.24.1.png|thumb|center|720x720px|'''Fig.18.1.''' Copy out, paste in (DDE). '''Left column'''. Illustrates the copy out paste in the transposition mechanism. The transposon is represented as a yellow line. Flanking sequences in the donor molecule are blue. Flanking sequences in the target molecule are green. Red circles indicate 3′OH moieties generated by Tpase-catalyzed hydrolysis at the transposon end(s). Red boxes indicate target DNA flanks that are duplicated on insertion. Top to bottom: Tpase catalyzed cleavage at the 3′ transposon ends using H2O as the nucleophile. Liberated 3′OH attack the opposite end to create a bridged molecule which then undergoes replication (dotted line) to generate a circular transponso copy and regenerate the donor molecule. The circle intermediate undergoes cleavage to generate two 3'OH ends which attack the target DNA in a staggered way, resulting in integration. Repair at each end then generates the direct target repeat characteristic of many transposons. | </nowiki></ref>; [https://tncentral.ncc.unesp.br/ISfinder/scripts/ficheIS.php?name=IS1R IS''1''] or [https://tncentral.ncc.unesp.br/ISfinder/scripts/ficheIS.php?name=IS2 IS''2''] insertion upstream of AcrEF<ref name=":7"><pubmed>11302812</pubmed> | ||
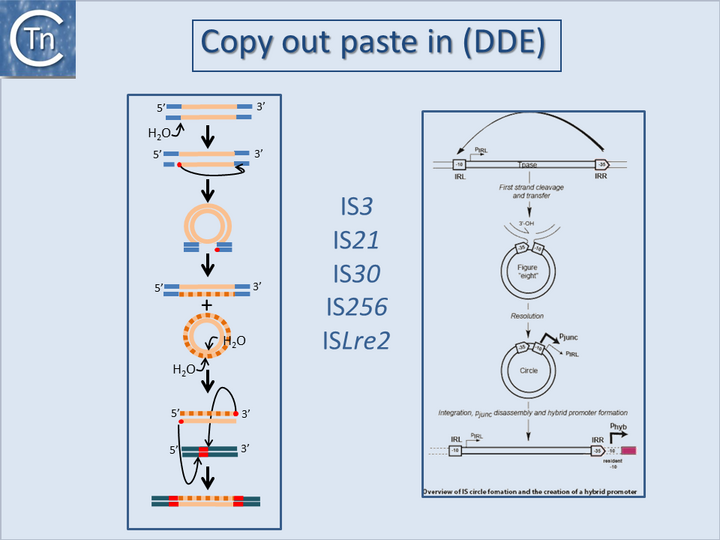
</nowiki></ref><ref><nowiki><pubmed>11274125</pubmed></nowiki></ref> and [https://tncentral.ncc.unesp.br/ISfinder/scripts/ficheIS.php?name=IS186 IS''186''] insertional inactivation of the AcrAB repressor, AcrR, in ''[[wikipedia:Escherichia_coli|Escherichia coli]] <ref name=":7" />'', all lead to increased resistance to [[wikipedia:Quinolone_antibiotic|fluoroquinolones]]. Insertional inactivation of specific [[wikipedia:Porin_(protein)|porins]] can also play a significant role<ref><nowiki><pubmed>15212803</pubmed></nowiki></ref>.<center>[[Image:1.24.1.png|thumb|center|720x720px|'''Fig.18.1.''' Copy out, paste in (DDE). '''Left column'''. Illustrates the copy out paste in the transposition mechanism. The transposon is represented as a yellow line. Flanking sequences in the donor molecule are blue. Flanking sequences in the target molecule are green. Red circles indicate 3′OH moieties generated by Tpase-catalyzed hydrolysis at the transposon end(s). Red boxes indicate target DNA flanks that are duplicated on insertion. Top to bottom: Tpase catalyzed cleavage at the 3′ transposon ends using H2O as the nucleophile. Liberated 3′OH attack the opposite end to create a bridged molecule which then undergoes replication (dotted line) to generate a circular transponso copy and regenerate the donor molecule. The circle intermediate undergoes cleavage to generate two 3'OH ends which attack the target DNA in a staggered way, resulting in integration. Repair at each end then generates the direct target repeat characteristic of many transposons. | |||
'''Right column'''. Formation of hybrid promoters by copy out -paste in transposition. The left and right terminal inverted repeats of the IS, IRL ,and IRR, are shown as a square and pointed box respectively. The component –10 and –35 promoter elements of p<sub>junc</sub> within these ends are also shown, together with the weak p<sub>IRL</sub> promoter and the direction of transcription of the transposase. In a first step, single-strand cleavage and transfer from one IR to the other is catalyzed by OrfAB produced from the weak p<sub>IRL</sub> promoter. This is indicated at the top of the figure by the curved arrow. In this case, the right end (IRR) is shown attacking the left end (IRL). The resulting figure-eight form is drawn below and shows the free 3’OH group generated on the flanking donor DNA sequence. In a second step, second-strand circularization occurs by an as yet undetermined mechanism involving host functions but independently of transposon proteins [Turlan et al., 2000]. The resulting IRR-IRL junction carries suitably placed -35 and –10 hexamers, separated by a canonical 17 bp spacer and form a strong p<sub>junc</sub> (bold arrow) promoter able to promote high levels of production of IS''911'' proteins. Integration of the circle results in disassembly of the promoter restoring low levels of expression from p<sub>IRL</sub>. Insertion upstream of a resident -10 promoter element can bring the IR-associated -35 element at the correct distance to form a promoter and activate a downstream gene.|alt=]] | '''Right column'''. Formation of hybrid promoters by copy out -paste in transposition. The left and right terminal inverted repeats of the IS, IRL ,and IRR, are shown as a square and pointed box respectively. The component –10 and –35 promoter elements of p<sub>junc</sub> within these ends are also shown, together with the weak p<sub>IRL</sub> promoter and the direction of transcription of the transposase. In a first step, single-strand cleavage and transfer from one IR to the other is catalyzed by OrfAB produced from the weak p<sub>IRL</sub> promoter. This is indicated at the top of the figure by the curved arrow. In this case, the right end (IRR) is shown attacking the left end (IRL). The resulting figure-eight form is drawn below and shows the free 3’OH group generated on the flanking donor DNA sequence. In a second step, second-strand circularization occurs by an as yet undetermined mechanism involving host functions but independently of transposon proteins [Turlan et al., 2000]. The resulting IRR-IRL junction carries suitably placed -35 and –10 hexamers, separated by a canonical 17 bp spacer and form a strong p<sub>junc</sub> (bold arrow) promoter able to promote high levels of production of IS''911'' proteins. Integration of the circle results in disassembly of the promoter restoring low levels of expression from p<sub>IRL</sub>. Insertion upstream of a resident -10 promoter element can bring the IR-associated -35 element at the correct distance to form a promoter and activate a downstream gene.|alt=]] | ||
| Line 23: | Line 25: | ||
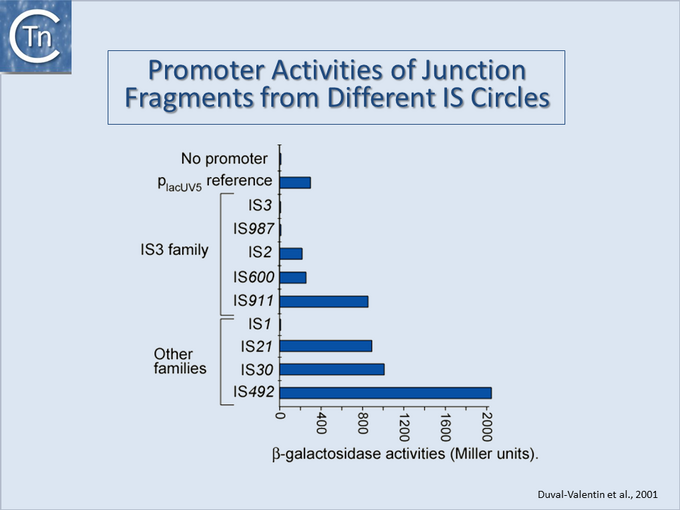
[[Image:1.24.2.png|thumb|center|680x680px|'''Fig.18.2.''' Promoter Activities of junction fragments from different IS circles. Results of measurements of activities of 9 IS circle junctions when placed upstream of a [[wikipedia:Beta-galactosidase|beta-galactosidase]] gene.|alt=]] | [[Image:1.24.2.png|thumb|center|680x680px|'''Fig.18.2.''' Promoter Activities of junction fragments from different IS circles. Results of measurements of activities of 9 IS circle junctions when placed upstream of a [[wikipedia:Beta-galactosidase|beta-galactosidase]] gene.|alt=]] | ||
</center><center> | </center><center></center> | ||
</center> | |||
==IS and Gene Expression== | ==IS and Gene Expression== | ||
{| class="wikitable" style="margin:auto" | {| class="wikitable" style="margin:auto" | ||
| Line 61: | Line 62: | ||
|[https://tncentral.ncc.unesp.br/ISfinder/scripts/ficheIS.php?name=ISKpn8 IS<i>Kpn8</i>]||Copy-paste circles||<i>blaKCP-2</i>||<i>[[wikipedia:Escherichia_coli|Escherichia coli]]</i>, <i>[[wikipedia:Citrobacter_freundii|Citrobacter freundii]]</i>, <i>[[wikipedia:Enterobacter_cloacae|Enterobacter cloacae]]</i>, <i>[[wikipedia:Klebsiella_aerogenes|Enterobacter aerogenes]]</i>, and <i>[[wikipedia:Klebsiella_oxytoca|Klebsiella oxytoca]]||<ref><nowiki><pubmed>24433026</pubmed></nowiki></ref>||C | |[https://tncentral.ncc.unesp.br/ISfinder/scripts/ficheIS.php?name=ISKpn8 IS<i>Kpn8</i>]||Copy-paste circles||<i>blaKCP-2</i>||<i>[[wikipedia:Escherichia_coli|Escherichia coli]]</i>, <i>[[wikipedia:Citrobacter_freundii|Citrobacter freundii]]</i>, <i>[[wikipedia:Enterobacter_cloacae|Enterobacter cloacae]]</i>, <i>[[wikipedia:Klebsiella_aerogenes|Enterobacter aerogenes]]</i>, and <i>[[wikipedia:Klebsiella_oxytoca|Klebsiella oxytoca]]||<ref><nowiki><pubmed>24433026</pubmed></nowiki></ref>||C | ||
|- | |- | ||
| rowspan="9" |[[IS Families/IS4 and related families|IS<i>4</i>]]|| rowspan="3" |[https://tncentral.ncc.unesp.br/ISfinder/scripts/ficheIS.php?name= | | rowspan="9" |[[IS Families/IS4 and related families|IS<i>4</i>]]|| rowspan="3" |[https://tncentral.ncc.unesp.br/ISfinder/scripts/ficheIS.php?name=IS10L IS<i>10</i>]||Cut-paste (hairpin)||<i>his</i>||<i>[[wikipedia:Salmonella_enterica_subsp._enterica|Salmonella typhimurium]]</i>||<ref><nowiki><pubmed>6289329</pubmed></nowiki></ref>||E | ||
|- | |- | ||
|Cut-paste||—||<i>[[wikipedia:Escherichia_coli|Escherichia coli]]</i>||<ref><nowiki><pubmed>6311437</pubmed></nowiki></ref>||E | |Cut-paste||—||<i>[[wikipedia:Escherichia_coli|Escherichia coli]]</i>||<ref><nowiki><pubmed>6311437</pubmed></nowiki></ref>||E | ||
| Line 67: | Line 68: | ||
|Cut-paste||<i>acrEF</i> pump||<i>[[wikipedia:Salmonella_enterica|Salmonella enterica]]</i>||<ref name=":6" />||E | |Cut-paste||<i>acrEF</i> pump||<i>[[wikipedia:Salmonella_enterica|Salmonella enterica]]</i>||<ref name=":6" />||E | ||
|- | |- | ||
|[https://tncentral.ncc.unesp.br/ISfinder/scripts/ficheIS.php?name= | |[https://tncentral.ncc.unesp.br/ISfinder/scripts/ficheIS.php?name=IS50R IS<i>50</i>]||Cut-paste (hairpin)||<i>aph3’II</i>||<i>[[wikipedia:Escherichia_coli|Escherichia coli]]</i>||<ref><nowiki><pubmed>6260374</pubmed></nowiki></ref>||E | ||
|- | |- | ||
| rowspan="2" |[https://tncentral.ncc.unesp.br/ISfinder/scripts/ficheIS.php?name= | | rowspan="2" |[https://tncentral.ncc.unesp.br/ISfinder/scripts/ficheIS.php?name=IS10A IS<i>1999</i>]|| rowspan="2" |—||<i>bla<sub>VEB-1</sub></i>||<i>[[wikipedia:Pseudomonas_aeruginosa|Pseudomonas aeruginosa]]</i>||<ref name=":2" />||C | ||
|- | |- | ||
|<i>bla<sub>VEB-1</sub></i>/<i>bla<sub>OXA-48</sub></i>||<i>[[wikipedia:Escherichia_coli|Escherichia coli]]</i>||<ref name=":1" />||C | |<i>bla<sub>VEB-1</sub></i>/<i>bla<sub>OXA-48</sub></i>||<i>[[wikipedia:Escherichia_coli|Escherichia coli]]</i>||<ref name=":1" />||C | ||
| Line 103: | Line 104: | ||
|<i>bla<sub>SHV-2a</sub></i>||<i>[[wikipedia:Pseudomonas_aeruginosa|Pseudomonas aeruginosa]]</i>||<ref><nowiki><pubmed>10223953</pubmed></nowiki></ref> | |<i>bla<sub>SHV-2a</sub></i>||<i>[[wikipedia:Pseudomonas_aeruginosa|Pseudomonas aeruginosa]]</i>||<ref><nowiki><pubmed>10223953</pubmed></nowiki></ref> | ||
|- | |- | ||
|[https://tncentral.ncc.unesp.br/ISfinder/scripts/ficheIS.php?name= | |[https://tncentral.ncc.unesp.br/ISfinder/scripts/ficheIS.php?name=IS26 IS<i>140</i>]||—||<i>aac(3)</i>-III and -IV||—||<ref><nowiki><pubmed>6318050</pubmed></nowiki></ref> | ||
|- | |- | ||
| rowspan="2" |[[IS Families/IS21 family|IS<i>21</i>]] | | rowspan="2" |[[IS Families/IS21 family|IS<i>21</i>]] | ||
Revision as of 12:59, 9 August 2021
Another important aspect of IS impact on their bacterial hosts is their ability to modulate gene expression. In addition to acting as vectors for gene transmission from one replicon to another in the form composite transposons (two IS flanking any gene; Fig.2.3) and tIS (Fig.8.1) and their ability to interrupt genes, it has been known for some time[1][2] that IS can also activate gene expression. This capacity has recently received much attention due to the increase in resistance to various antibacterials[3][4][5], a worrying public health threat[6][7].
They can accomplish this in two ways: either by providing internal promoters whose transcripts escape into neighboring DNA[2][8][9][10] or by hybrid promoter formation. Many IS carry -35 promoter components oriented towards the flanking DNA (Fig.18.1). In a number of cases this plays an important part in their transposition since a significant number of IS transposes using an excised transposon circle (Fig.18.1) with abutted left and right ends. For these IS, the other end carries a -10 element oriented inwards towards the Tpase gene. Together with the -35, this generates a strong promoter on formation of the circle junction to drive Tpase expression required for catalysis of integration (Fig.18.2) [11][12][13][14]. Thus, if integration occurs next to a resident -10 sequence, the IS -35 sequence can contribute to a hybrid promoter to drive expression of neighboring genes [see [15]]. At present, this phenomenon had been reported to occur with over 30 different IS in more than 17 bacterial species[16][17] (Table IS and Gene Expression below). Indeed, specific vector plasmids have been designed to identify activating insertions (e.g. [18]).
IS activity can affect efflux mechanisms resulting in increased resistance: IS1 or IS10 insertion can up-regulate the AcrAB-TolC pump in Salmonella enterica[19]; IS1 or IS2 insertion upstream of AcrEF[20][21] and IS186 insertional inactivation of the AcrAB repressor, AcrR, in Escherichia coli [20], all lead to increased resistance to fluoroquinolones. Insertional inactivation of specific porins can also play a significant role[22].
IS and Gene Expression
Bibliography
- ↑ <pubmed>1101028</pubmed>
- ↑ 2.0 2.1 2.2 2.3 <pubmed>6271458</pubmed> </nowiki>
- ↑ 3.0 3.1 <pubmed>16952941</pubmed> </nowiki>
- ↑ 4.0 4.1 <pubmed>12923109</pubmed> </nowiki>
- ↑ 5.0 5.1 5.2 5.3 5.4 <pubmed>23158541</pubmed> </nowiki>
- ↑ <pubmed>23887414</pubmed>
- ↑ <pubmed>23887415</pubmed>
- ↑ 8.0 8.1 <pubmed>6292860</pubmed> </nowiki>
- ↑ <pubmed>6260746</pubmed>
- ↑ <pubmed>6311437</pubmed>
- ↑ <pubmed>26350305</pubmed>
- ↑ <pubmed>9214651</pubmed>
- ↑ <pubmed>10438765</pubmed>
- ↑ <pubmed>11598022</pubmed>
- ↑ 15.0 15.1 <pubmed>3029382</pubmed> </nowiki>
- ↑ <pubmed>17223624</pubmed>
- ↑ <pubmed>24499397</pubmed>
- ↑ <pubmed>8863735</pubmed>
- ↑ 19.0 19.1 19.2 <pubmed>15616308</pubmed> </nowiki>
- ↑ 20.0 20.1 20.2 <pubmed>11302812</pubmed> </nowiki>
- ↑ <pubmed>11274125</pubmed>
- ↑ <pubmed>15212803</pubmed>
- ↑ 23.0 23.1 23.2 <pubmed>6283551</pubmed></nowiki>
- ↑ <pubmed>4610339</pubmed>
- ↑ <pubmed>339095</pubmed>
- ↑ <pubmed>6187472</pubmed>
- ↑ <pubmed>22992527</pubmed>
- ↑ <pubmed>12867459</pubmed>
- ↑ <pubmed>15130120</pubmed>
- ↑ <pubmed>24433026</pubmed>
- ↑ <pubmed>6289329</pubmed>
- ↑ <pubmed>6311437</pubmed>
- ↑ <pubmed>6260374</pubmed>
- ↑ 34.0 34.1 <pubmed>12936998</pubmed></nowiki>
- ↑ 35.0 35.1 <pubmed>12951337</pubmed></nowiki>
- ↑ <pubmed>14742218</pubmed>
- ↑ <pubmed>16441449</pubmed>
- ↑ <pubmed>16630258</pubmed>
- ↑ 39.0 39.1 <pubmed>19749055</pubmed></nowiki>
- ↑ <pubmed>7568465</pubmed>
- ↑ <pubmed>8067736</pubmed>
- ↑ 42.0 42.1 <pubmed>8057831</pubmed></nowiki>
- ↑ <pubmed>7545155</pubmed>
- ↑ 44.0 44.1 44.2 <pubmed>3025189</pubmed></nowiki>
- ↑ <pubmed>7840551</pubmed>
- ↑ <pubmed>10852863</pubmed>
- ↑ <pubmed>18443121</pubmed>
- ↑ <pubmed>2160941</pubmed>
- ↑ <pubmed>10223953</pubmed>
- ↑ <pubmed>6318050</pubmed>
- ↑ <pubmed>7517394</pubmed>
- ↑ <pubmed>18227185</pubmed>
- ↑ <pubmed>3039299</pubmed>
- ↑ <pubmed>9756793</pubmed>
- ↑ <pubmed>23014718</pubmed>
- ↑ <pubmed>3038844</pubmed>
- ↑ <pubmed>9371438</pubmed>
- ↑ <pubmed>12511511</pubmed>
- ↑ <pubmed>9098071</pubmed>
- ↑ <pubmed>7830550</pubmed>
- ↑ <pubmed>27047473</pubmed>
- ↑ 62.0 62.1 62.2 62.3 <pubmed>11344163</pubmed></nowiki>
- ↑ 63.0 63.1 63.2 63.3 <pubmed>12604530</pubmed> </nowiki>
- ↑ <pubmed>8602160</pubmed>
- ↑ <pubmed>19744834</pubmed>
- ↑ <pubmed>11470367</pubmed>
- ↑ <pubmed>12435670</pubmed>
- ↑ <pubmed>16569841</pubmed>
- ↑ <pubmed>16940134</pubmed>
- ↑ <pubmed>9202480</pubmed>
- ↑ <pubmed>9765560</pubmed>
- ↑ <pubmed>20520730</pubmed>